
Short-hairpin RNA (shRNA) is an RNA molecule that contains sense and antisense sequences connected by a short spacer of nucleotides that enables the molecule to form a loop structure. shRNA is expressed in mammalian cells from a vector with a Pol III-type promoter. It is transported from the nucleus into the cytoplasm, where it's processed by the Dicer enzyme into 21-23 nucleotide siRNA duplexes. Once in the cell, the shRNA can enter the RNAi pathway and result in gene silencing by blocking translation.
The BLOCK-iT RNAi Designer can design double-stranded DNA (dsDNA) molecules for directional cloning into a Gateway®-adapted entry vector and subsequent expression of shRNA. The dsDNA has complementary 21-mer sequences and a default 4-nucleotide loop sequence or a custom loop sequence that you specify and a 4-nucleotide sequence (CACC or AAAA) for directional cloning. An example of a dsDNA design for encoding shRNA is shown below:

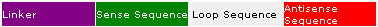
To begin designing a dsDNA oligo, select the shRNA target design option at the top of the Designer page. Then enter a sequence for the gene that you want to target or a database accession number for that sequence, select a database to BLAST search to identify unique regions of the sequence, and specify a GC percentage range for the oligos. Finally, select the expression vector and the strand orientation of the oligo.
You can copy and paste a nucleotide target sequence directly into the Nucleotide Sequence field, or you can enter a database accession number for the sequence in the Accession Number field. If you enter an accession number, the Designer will link to the Entrez search engine and search the crosslinked databases at the National Center for Biotechnology Information (NCBI), including GenBank and OMIM. Then the sequence associated with the accession number will be automatically downloaded into the Designer.
Note: If you enter an accession number in RefSeq format (e.g., NT_123456), the Designer will check the sequence for known single-nucleotide polymorphisms (SNPs). Designs that include SNPs will be rejected by the Designer. SNP identification will not be performed if you paste in nucleotide sequences or enter GenBank-format accession numbers.
Nucleotide Sequence: Type or paste your single-stranded target sequence into this box. The designer accepts sequences in text, FASTA, or GenBank format.
The sequence should be oriented from 5' to 3'. Because shRNA molecules must be highly specific, the input sequence should contain only the following 1-letter base code abbreviations: A, C, G, T, U (case insensitive). Any regions of the sequence containing ambiguous bases (e.g., N) or other letters will be skipped when generating the designs. If your sequence includes too many ambiguous bases or other letters, you will be prompted to re-enter the sequence. White spaces and numerals in the sequence will be ignored.
Note: If you are having difficulty generating designs, check your input sequence to make sure that it contains only the letters A, C, G, T, or U.
Accession Number: Enter the database accession number for your sequence in this field. The Designer will link to the Entrez search engine and search the crosslinked databases at NCBI, including GenBank and OMIM. The sequence associated with the accession number will be automatically downloaded into the Designer.
RefSeq-format accession numbers begin with two letters followed by an underscore and six digits (e.g., NT_123456). GenBank accession numbers are usually a combination of a letters and numbers, such as a single letter followed by five digits (e.g., U12345) or two letters followed by six digits (e.g., AF123456). If you enter an accession number in RefSeq format (e.g., NT_123456), the Designer will check the sequence for known single-nucleotide polymorphisms (SNPs). Designs that include SNPs will be rejected by the Designer. SNP identification will not be performed if you paste in nucleotide sequences or enter GenBank-format accession numbers.
If you entered an accession number in Step 1, in this step you specify the regions of the sequence for which you want to design your dsDNA molecules. The default selection of Open Reading Frame (ORF) is appropriate in most cases. If you have trouble generating designs for the ORF alone, select the 5' UTR and/or 3' UTR checkboxes and redesign the molecules.
Step 3. Choose Database for BLAST
In Step 3, select a species-specific database from the pulldown list that you want to perform a BLAST (Basic Local Alignment Search Tool) search on to identify unique regions in your target sequence. Areas of homology identified by the BLAST search will be "masked" in the sequence to ensure that the target designs are highly specific.
Each BLAST search is performed against a a nonredundant version of the UniGene database. The database contains representative gene sequences for the selected species.
Note: The databases used in this search contain one representation of each gene and do not include splice variants.
The Minimum GC Percentage and Maximum GC Percentage fields contain default values for the minimum and maximum percentage GC content of your shRNA molecules. For most sequences, this range is wide enough to generate several designs.
You can change this range if you are having difficulty generating designs, or if you know that the GC content of your dsDNA molecules will fall outside this range based on the sequence. Designs outside the selected range will be rejected by the Designer.
In this step, you select the Gateway®-adapted entry vector for expressing your shRNA and the strand encoding the shRNA, and then click on the RNAi Design button. Up to 10 designs will be generated for your target sequence.
Select the desired vector from the Vector pulldown list. Select either the pENTR™/U6 entry vector for constitutive shRNA expression or the pENTR™/H1/TO entry vector for inducible expression. Note that if you select pENTR™ /U6, the shRNA oligo designs will be compatible with both vectors.
Next, select the desired Strand Orientation, depending on whether the resulting shRNA should be encoded from the sense target sequence (sense-loop-antisense) or the antisense target sequence (antisense-loop-sense).
When you have made your selections, click on the RNAi Design button.
For information about design results, see shRNA Design Results.